|
| Background for the student .
. . . .
Diprotic acids are substances (molecules) that have two protons that can be released in water. A very common diprotic acid is sulfuric acid, H2SO4. In water, both protons of sulfuric acid are released, giving hydrated protons 2H+(aq) and sulfate dianion, SO4 (2-):
The pH of the solution, since it is very acidic, is low. (The number is well below zero!) Carbonated water (club soda) is another example of a diprotic acid. This diprotic acid and its monoprotic acid partner are formed when carbon dioxide is bubbled through water.
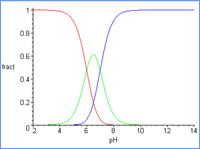
In a general case as the pH of an aqueous solution increases, a diprotic acid will ionize first to a monoprotic acid ( ha- ) and one proton ( H+ ) and then the monoprotic acid ( ha- ) will ionize to a dianion ( a= ) and liberate the second proton ( H+ ). |
 |
|
||
 As the pH increases (horizontal axis), the fraction of the diprotic acid ( yellow curve ) decreases while the fraction of the monoprotic anion ( green curve ) increases. Then, above pH = 8, the dianion ( red curve representing no protons ) forms as the monoprotic acid (green curve) is depleted. |
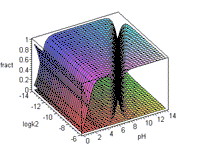
Study the curves on the left (below) and then click on the graph to see the animation. These exercises use examples of acids found in chemical and biochemical systems.
|
Any National Curve Bank deposit on
acid-base reactions would not be complete without
acknowledging Dr. Arnold O. Beckman, the inventor
of the pH meter and a major contributor to the
technology revolution of the 20th century.
This photo of Dr.Beckman was taken on his 100th birthday, April 10, 2000, at Casa Pacifica, San Clemente, California. In addition to his many other accomplishments - scholar, inventor, civic leader and philanthropist - he is one of the U.S.'s oldest living Marines. Arnold has several rules for living a good life. One is always do your best. Never do anything half-heartedly. |
 |
| Zaven Karian at Denison University
in Granville, Ohio brought together faculty from
across liberal arts departments to see what they
might do with computational tools using Maple
software. Hoffman's pH animations
are one outcome. The project was supported by
the Fund for Improvement of Post Secondary Education
(FIPSE) of the Department of Education (Grant
#P116B30079).
The NCB thanks Hoffman and Karian for their fine efforts. Moreover, we thank Jason Schattman of Waterloo Maple, Inc. for calling this work to our attention. This deposit represents a merger of applied mathematics with chemistry using computer software. |
| For the Maple code that created the animations please see < http://www.mapleapps.com/categories/science/chemistry/html/Ab6.html > |